Abstract
Introduction:
There are scarce data regarding treatment outcomes and toxicity in Latin American countries. Argentina is the second largest country in the region and the fourth most populated one. National Guidelines from the Argentinean Society of Hematology (SAH) recommends the use of bortezomib based triplets for induction treatment in transplant eligible newly diagnosed Multiple Myeloma patients.
Objective:
To compare response rates and adverse events after induction treatment with Cyclophosphamide Bortezomib and Dexamethasone (CyBorD) or Bortezomib Thalidomide and Dexamethasone (VTD) outside of clinical trials in a Latin American country.
Methods:
Retrospective multicentric cohort study. All centers participating in the Argentinean Multiple Myeloma Study Group (GAMM) were invited to participate in the study.
Eligible patients were 75 years of age or younger, with a diagnosis of Multiple Myeloma according to the IMWG 2014 criteria, transplant eligible, treated with at least one cycle of CyBorD or VTD as induction therapy in the time period from December 2012 until December 2017. Main exclusion criteria were amyloidosis, plasma cell leukemia and previous neuropathy.
Patients were identified from local registries at each center and included consecutively in the study database. Epidemiological and clinical data were obtained from medical records and collected in a standardized clinical report form. Patients were followed from diagnosis until death or lost to follow up. Response was evaluated according to IMWG Response Criteria 2016. Adverse events were graded by CTCAE 4.3.
Comparisons of response rates were performed using a Chi2 test and differences in rates were expressed as proportions with 95% confidence intervals (CI). Crude odds ratios (OR) and OR adjusted by potential confounders were calculated using a logistic regression model. Kaplan Meier method was used to estimate progression free survival (PFS) and overall survival (OS). Stata 13 software was used.
Results:
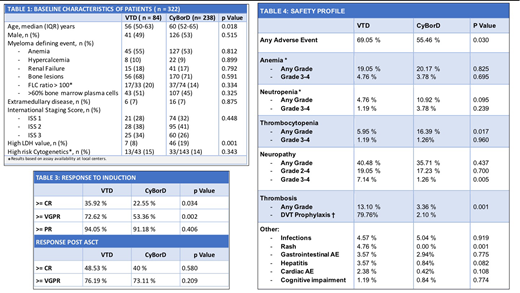
A total of 322 patients from 15 centers in Argentina were included in the study. The median age at diagnosis was 57 years (range 26-74), 52% (167) of the patients were male, 18% (58) had renal failure, 28% (85) ISS 3 , 7% (22) extramedullary disease, and 14% (46) high risk cytogenetics. Median time of follow up was 34 months (IQR 21-58).
CyBorD was the most common treatment, indicated as induction therapy in 74% (238) of the cases. The characteristics of the patients were similar in both groups except age and LDH levels.
The median number of cycles was 5 (range 1-12). Bortezomib was administered once per week in 85% (272) of the patients and subcutaneously in 86% (276) with no differences between both treatment arms. The median cumulative cyclophosphamide dose per month was 1.5 g (IQR 1.5-2.4) and thalidomide dose per day was 100 mg.
In the VTD arm, 72,62% (61) of the patients achieved at least very good partial response (VGPR) vs 53.36% (127) with CyBorD [OR of 2.31 (CI 1.35 - 3.99) p=0.002]. The difference in VGPR was 19.26% (CI 15 - 24). Complete response rate (CR) was 35.92% in patients treated with VTD vs 22.55% with CyBorD [OR of 1.87 (CI 1.04 - 3.35) p=0.03). The difference in CR was 13,37% (CI 9.6 -17.53). There was no difference in overall response rate (ORR) with 94.05% vs 91.18% (p=0.406).
Adverse events were more common with VTD (69.05% vs 55.46% p=0.030), especially neuropathy grade 3 - 4 (7.14% vs 1.26% p=0.005) and thrombosis (13.10 % vs 3.36 % p=0.001). Deep venous thrombosis prophylaxis was inadequate in 20.24% of the patients. Hematologic adverse events were more common with CyBorD, especially thrombocytopenia (5.95% vs 16.39% p=0.017).
Autologous stem cell transplantation (ASCT) was performed in 78% (249) of patients. There was 5% (17) stem cell mobilization failure, all in the CyBorD arm. Response rates after ASCT with VTD and CyBorD induction treatment were: 76.19 vs 73.11% VGPR (p=0.580) and 48.53% vs 40% CR (p=0.20). Maintenance treatment was indicated in 67.86% (57) and 65.13% (155) patients respectively (p=0.650).
The PFS at 24 months was 83% (CI 71-90) with VTD vs 72% (CI 66-78) [(HR 0.92 (CI 0.59 - 1.42) p 0.715] and OS 96% (CI 87-99) vs 91% (86-94) respectively [(HR 1.2 (CI 0.62 - 2.32) p 0.587].
Conclusions:
VTD has better CR and VGPR compared to CyBorD. Nevertheless, CyBorD continues to be the preferred induction regimen in Argentina based on safety profile. The optimal number of induction treatment cycles remains to be determined.
Schutz:Takeda: Honoraria, Research Funding; Sanofi Aventis: Research Funding; Roche: Research Funding; Glaxo: Research Funding; Janssen: Honoraria, Research Funding; Varifarma: Honoraria. Shanley:Brystol Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Fantl:Janssen: Consultancy, Honoraria, Research Funding; Varifarma/Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Research Funding; Roche: Research Funding; Tecnofarma: Honoraria; BMS: Consultancy, Honoraria; Glaxo: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal